Abstract
Introduction: Sickle cell disease (SCD) is characterized by acute vaso-occlusive events and chronic hemolytic anemia, originating from the polymerization of the abnormal hemoglobin S (HbS) under low oxygen tension. Hemolysis is defined as an imbalance between the rate of red blood cell (RBC) production and destruction. It results in an overall reduced lifespan of RBCs, as they are prematurely removed from the circulation. While the normal RBC lifespan is approximatively 120 days, those of SCD is only a sixth of it (Hebbel, 2011). Fetal Hb (HbF) is the main clinical modulator of SCD severity by inhibiting HbS polymerization. Its protective effect relies on its quantity and distribution among the RBC population. However, HbF is often heterogeneously distributed in RBC from SCD patients. Among the numerous improvements triggered by HbF, a significant increase in RBC lifespan has been measured in high-HbF containing cells, as compared to low-HbF containing cells (Franco et al., 2006).
As they age, normal RBCs exhibit physiological changes, such as dehydration, a decrease of the intracellular enzymes activities (pyruvate kinase and hexokinase) and an increase in Hb concentration. Glycated Hb (the most abundant being HbA1c form) also increases, as RBC age. The glycation of Hb is a non-enzymatical reaction that is affected by the plasmatic concentration of glucose and the time RBCs last in the circulation. In non-diabetic subjects, the Hb glycation only depends on the RBC lifespan. To date, current methods to assess RBC lifespan are invasive and time consuming, requiring infusion of autologous labeled RBCs and long follow-up.
Objectives: This present work aims at 1/ evaluating the RBC relative age of SCD patients using glycated Hb measured by flow cytometry and 2/ estimating the HbF-induced increase in RBC lifespan of SCD patients.
Methods: Glycated Hb was measured and compared between five healthy donors (AA), seven non-diabetic and non-transfused homozygous SCD patients (SS) and five non-diabetic, transfused SS patients. Total glycated Hb was measured by reference biochemical methods, cation-exchange high-pressure liquid chromatography or rapid glycohaemoglobin immunoassay (DCA test) used for AA-RBC and SS-RBC, respectively. The single cell glycation was assessed by flow cytometry using mouse anti-human HbA1c antibody. HbA, HbS and HbF were assessed by flow cytometry using anti βA-globin, anti βS-globin and anti g-globin antibodies, respectively.
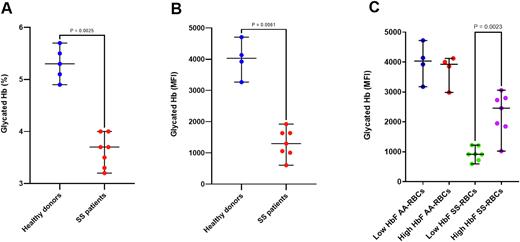
Results: The glycated Hb was significantly lower in SS patients than in healthy donors (glycated HbS =3.7%, glycated HbA =5.3%, p=0.0025 by DCA and HPLC respectively, and by flow cytometry p=0.0061). In transfused SS patients, results showed that glycated Hb was lower in autologous SS-RBCs than in AA-RBCs by a factor of four (P= 0.008), thus confirming the characterized reduced lifespan of SS RBCs. Glycated Hb levels were measured in RBC sub-populations based on their HbF level (high HbF or low HbF subfractions) in non-transfused SS patients. Glycated Hb level was lower in low HbF RBCs, as compared to high HbF RBCs (P=0.0023). This difference was not detected in healthy donors, thereby indicating that HbF has an effect on relative age only in SCD patients.
Conclusion: Single cell glycated Hb assessed by flow cytometry allows to simultaneously estimate the relative age of different RBC sub-populations. This new tool is noninvasive and requires only one single time point measurement. It should also be considered to evaluate the efficacy of treatments that aim at improving hemolysis.
Figure. Glycated Hb levels assessed by HPLC and DCA biochemical methods, and flow cytometry in different participant groups and RBC subpopulations. Results were expressed as median, percentage or mean fluorescence intensity (MFI) when appropriate. Glycated Hb level was compared between two groups using a nonparametric Mann-Whitney t-test. (A) Total glycated Hb measured by HPLC in AA healthy donors (n=5) and by DCA test in SS patients (n=7). (B) Single cell glycated Hb measured by flow cytometry in SS RBCs (n=7), compared to AA healthy donors (n=4). (C) Single cell glycated Hb measured in low HbF RBCs (n=7) and high HbF RBCs (purple, n=7) subpopulations in SS patients and AA healthy donors (n=4).
Disclosures
Djouder:AddMedica: Research Funding. Keumeugni:AddMedica: Current Employment. Duguet:AddMedica: Current Employment. Bartolucci:Bluebird bio: Consultancy, Research Funding; Emmaus: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Global Blood Therapeutics: Consultancy, Research Funding; Hemanext Inc: Consultancy; Novartis: Consultancy, Research Funding; Innovhem: Other: Co-founder; JazzPharma: Consultancy; Fabre Foundation: Research Funding; Roche: Consultancy; Addmedica: Consultancy, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal